Electromagnetic Radiation
Course Detail
Electromagnetic Radiation
Introduction
Electromagnetic radiation (EM radiation or EMR) refers to the waves of the electromagnetic field (photons or light quanta), propagating (radiating) at the universal speed of light through free space or through a material medium, carrying electromagnetic radiant energy. It includes radio and TV waves, radar waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays.
Visible light is a form of electromagnetic radiation. This theory was proved by Thomas Young (1801) by passing light through a barrier with slits, it was observed that each slit acted as a point-source and waves from each source interfered with each other.
Maxwell's Equations
In 1865, James Maxwell developed four equations that showed that electric and magnetic fields were linked and that the fields can move through space as waves.
1. Electric field lines begin and end on electric charges
Electric field lines originate on positive charges and terminate on negative charges. Maxwell's first equation can be used to develop a special form of Coulomb's law known as Gauss's law for electricity.
2. Magnetic field lines are continuous with no beginning nor ending:
There are no magnetic monopoles. The strength of the magnetic field is related to the magnetic constant (also known as the permeability of free space). This equation is also known as Gauss's law of magnetism.
3. A changing electric field will produce a magnetic field:
This encompasses Ampere's law.
4. A changing magnetic field will produce an electric field:
A changing magnetic field induces an electromotive force which results in an electric field. This equation encompasses Faraday's law of induction and also included Lenz's law.
Properties of Electromagnetic Waves
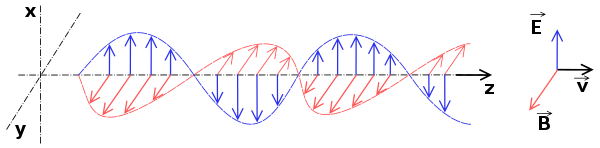
Electromagnetic waves are produced by accelerating electric charges, the accelerated charges lose energy, which is carried away as a wave.
If the charge is accelerated in simple harmonic motion, the frequency of the wave will be equal to the frequency of the oscillating charge.
The changing fields will travel through space at the speed of light i.e., 3.00x108 m/s.
The magnetic field is at right angles to the electric field and both are at right angles to the direction of travel.
Light and other electromagnetic waves consist of oscillating electric and magnetic fields moving together.
All electromagnetic waves show wave properties including reflection, refraction, diffraction and interference.
The Electromagnetic Spectrum
Heinrich Hertz was first to report the presence of visible electromagnetic waves. Using a reflector and measuring the wavelength, Hertz was able to calculate the speed of light.
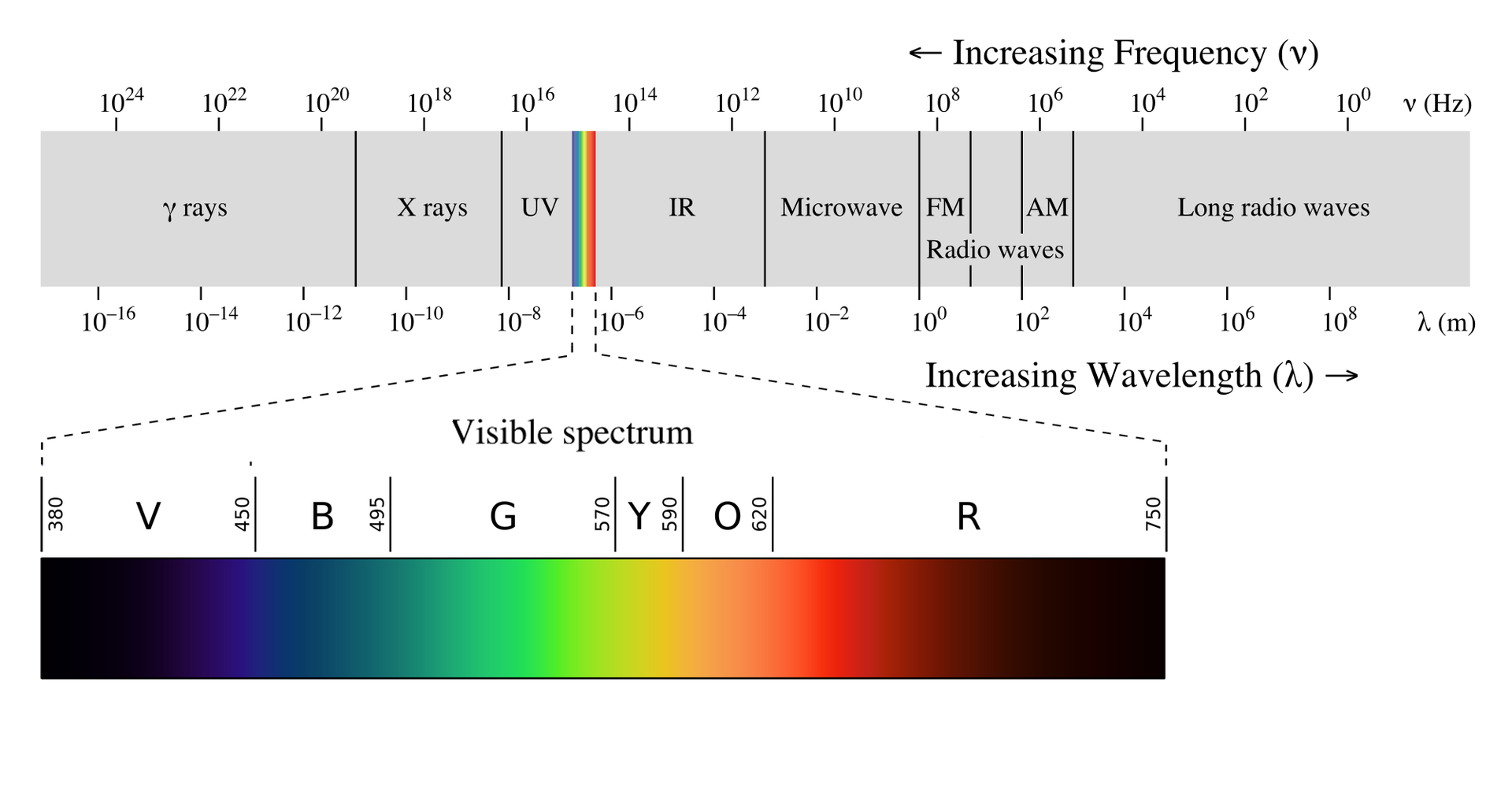
Long radio waves
These include radio waves. They have a large wavelength of km and therefore have low frequency. They are produced by accelerating electric currents such as AC power lines and are used in special communications.
Radio Waves
Their wavelengths range from hundreds of meters to a few meters. They are produced by accelerating electrons in conductors. They are also used in communication systems.
Radio waves can be categorized into several types depending on their wavelengths as follows:
- UHF - Ultra High Frequency: used for televisions, cell phones, satellite GPS, WiFi, Bluetooth, Walkie Talkies, cordless phones etc.
- VHF - Very High Frequency: used in FM radios, television broadcasting, mobile radio systems like emergency and military, marine communications, air traffic control etc.
- HF - High Frequency: This is a major part of the short-wave band frequencies used in short wave radio. These waves are best for long distance communication, so they are used across intercontinental distances and for mountainous terrains. Shortwaves are also used in aviation communication, government time stations, weather stations etc.
- MF - Medium Frequency: This includes the AM broadcast band most commonly used in AM radio broadcasting, navigational radio beacons, maritime communications etc.
- LF - Low Frequency: these are suitable for long distance communications because they experience low signal attenuation. In some parts, the high side of this band can be used for AM broadcasting. Other uses include navigation, weather systems and aircraft beacons.
- VLF - Very Low Frequency: These wavelengths are limited in audio transmission, but they have the advantage of high penetrance in water, so they are used for military communication.
- ULF: Ultra Low Frequency: These waves are released by the magnetosphere and they represent important physical processes in the near-earth plasma environment. The band is often used in mines and it can penetrate the earth. Some studies have reported that some earthquakes are preceded by an increase in ULF activity.
- SLF - Super Low Frequency: These frequencies are difficult to generate artificially, but they can penetrate seawater to depths of hundreds of meters. Therefore, they have been used in some cases (Russia and India) to communicate with submarines.
- ELF - Extremely Low Frequency: These wavelengths are produced by lightning and other disturbances of the earth's magnetic field. they can also penetrate seawater making them suitable for submarine communications in countries including USA, Russia and India.
Microwaves
Their wavelength ranges from millimeters to centimeters. They are produced by special vacuum tubes and circuits. They are mostly used in the household appliances for heating. However, other uses include radar communication.
Infrared
Their wavelengths are in millimeters. They are produced by vibrating molecules and atoms (heat) and lasers. Infrared wavelengths have been used extensively for military and civilian applications including target acquisition, surveillance, night vision, homing, and tracking. Non-military uses include thermal efficiency analysis, environmental monitoring, industrial facility inspections, detection of grow-ops, remote temperature sensing, short-range wireless communication, spectroscopy, and weather forecasting.
Visible light
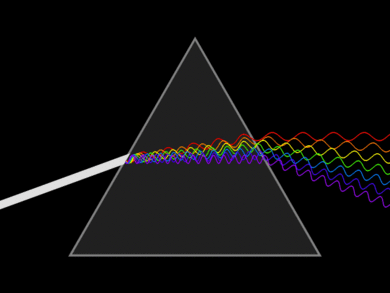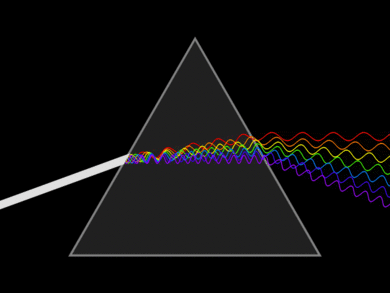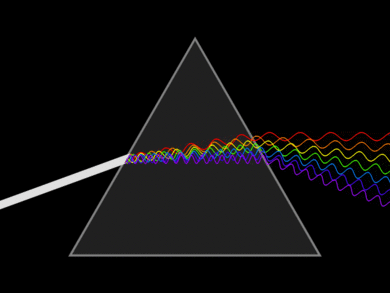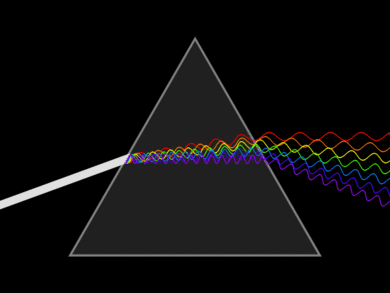
Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nm. The main source of light on Earth is the Sun. Sunlight provides the energy that green plants use to create sugars mostly in the form of starches, which release energy into the living things that digest them. Visible light can affect only a tiny percentage of all molecules. Usually not in a permanent or damaging way, rather the photon excites an electron which then emits another photon when returning to its original position. This is the source of color produced by most dyes. Retinal is an exception. When a photon is absorbed the retinal permanently changes structure from cis to trans, and requires a protein to convert it back, i.e. reset it to be able to function as a light detector again.
Ultraviolet
electromagnetic waves with a wavelength less than 400nm long. Produced by electrons dropping from very high energy levels to low energy levels. They are used for sterilizing materials, tanning, and generally in research. Ionizing radiations can knock electrons out of atoms and break bonds.
X- Rays
X-rays are produced by a sudden deceleration of very high-speed electrons. They are used in medical and industrial imaging. They are also ionizing radiations, this explains why X-rays can result in cancer, when they break bonds in DNA causing mutations or DNA damage.
Gamma Rays
Gamma rays are produced the decomposition of nuclei either spontaneously or by decelerating atomic nuclei. They are produced in nuclear reactions. They can penetrate matter very deep and can destroy carcinogenic or mutant cells hence they can be used in cancer treatment. They are ionizing radiations and can cause radiation sickness.
Light Reflection
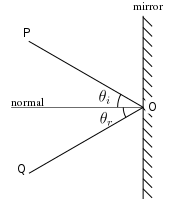
Light can be expressed using the ray or wave model. The law of light reflection states that the angle of reflection is equal to the angle of incidence and is in the same plane. Mirrors can be used to reflect light and observe an image or a real object. The image may appear the same size as the object, or magnified, it may appear erect (upright) or inverted, and it may be real or virtual image. Images formed by curved mirrors will depend on the angle of curvature. Concave mirrors are called converging mirrors while convex mirrors are called diverging mirrors.
Light Refraction
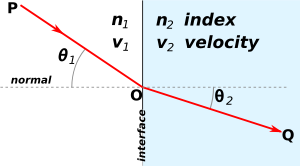
Light travels at different speeds in different media. When light strikes a medium in which it has a different speed, it bends. Refraction occurs when some of the incidence light does not enter the medium and it gets reflected, while some of the light enters the medium and refracts. The refraction depends on the difference in refractive indexes between the two media. The more the medium slows the light, the greater the index of refraction. When light enters a medium with a higher refraction index, it bends toward the normal. When light enters a medium with lower refraction index, it bends away from the normal.
Total internal reflection
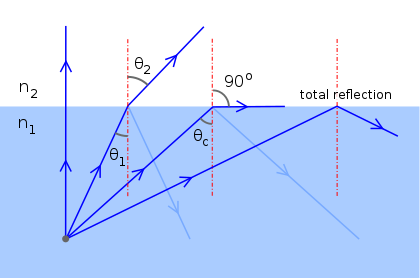
Total internal reflection occurs when light is moving into a higher index material and the incidence angle is large, in this case there is no refracted ray formed. The minimum incidence angle at which total internal reflection occurs is called Critical angle.
Light Dispersion
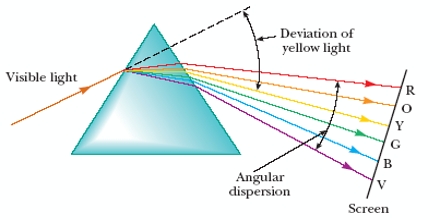
Light can be dispersed into a spectrum using refraction on a prism. Dispersion is the separation of white light into its spectrum colors. These colors form 6 bands in the order Red, Orange, Yellow, Green Blue and Violet (ROYGBV). Larger wavelength refracts less than lower wavelengths. Adding another prism on the other end can recombine the spectrum back into white light. This was demonstrated by Sir Isaac Newton.
Lenses
Lenses are used in several optical instruments, ranging from a simple magnifying glass to microscopes and cameras. the eye also contains a lens that function in the same way as other equipment. generally, there are two kinds of lenses, Diverging (Concave) and Converging (Convex) lens. Rays of light entering a converging lens or a diverging lens, parallel to its axis, converge at its focal point F. The distance from the center of the lens to the focal point is the lens’s focal length f.
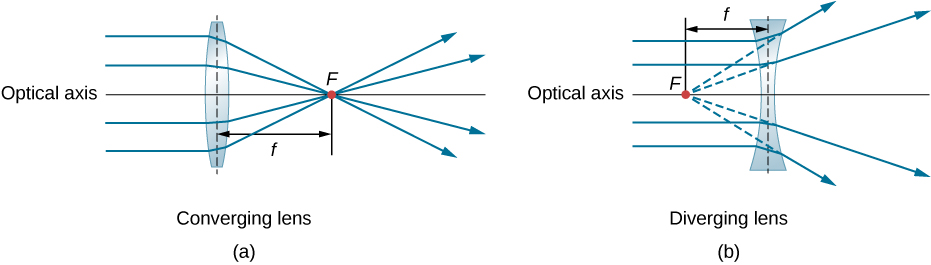
Diffraction and Interference
Waves can bend around corners and spread out again after passing through an opening in a barrier. This change in direction is called Diffraction.
Waves also exhibit interference. When waves add together, they result in constructive interference, and when waves subtract from each other they form destructive interference. Light also undergoes interference as proven by Thomas Young in 1801.
The Photoelectric Effect
In 1887, Heinrich Hertz showed that certain metals lost negative charge when exposed to UV light. The term photoelectric effect was coined to explain this phenomenon and the electrons given off were called photoelectrons. The photoelectrons were released if the incidence frequency was above a threshold frequency (fo). Each metal has its own threshold frequency. If the incident light was below the fo the photoelectrons will not be emitted. Increasing the light intensity results in a larger number of photoelectrons.
When the frequency of the incident light is increased, the maximum kinetic energy of the photoelectrons increases. When the intensity of light increases, the maximum kinetic energy does not change but the number of photoelectrons emitted increases.
Albert Einstein proposed that depending on the material, electrons require a minimum amount of energy to be freed from the atom. This can be referred to as Work function. The photoelectrons have maximum kinetic energy equal to the energy they gained from the photons subtract the work function.
Quantum Mechanics
Quantum Mechanics defines the present model of the atom, it incorporates the wave and particle nature of matter. Quantum effects are only noticeable at the atomic scale. The quantum theory also gives the number of electrons possible in each of the energy levels (and therefore the number of elements in each period of the periodic table).